Abstract
Traumatic brain injury (TBI) is a global problem and causes long-term disability in millions of individuals. This is a major problem for both military and civilian-related populations. The prevalence of sleep disorders in individuals with TBI is very high, yet mostly unrecognized. Approximately 46% of all chronic TBI patients have sleep disorders, which require nocturnal polysomnography and the Multiple Sleep Latency Test for diagnosis. These disorders include sleep apnoea (23% of all TBI patients), post-traumatic hypersomnia (11%), narcolepsy (6%) and periodic limb movements (7%). Over half of all TBI patients will have insomnia complaints, most often with less severe injury and after personal assault, and half of these may be related to a circadian rhythm disorder. Hypothalamic injury with decreased levels of wake-promoting neurotransmitters such as hypocretin (orexin) and histamine may be involved in the pathophysiology of excessive sleepiness associated with TBI. These sleep disorders result in additional neurocognitive deficits and functional impairment, which might be attributed to the original brain injury itself and thus be left without specific treatment.
Most standard treatment regimens of sleep disorders appear to be effective in these patients, including continuous positive airway pressure for sleep apnoea, pramipexole for periodic limb movements and cognitive behavioural therapy for insomnia. The role of wake-promoting agents and CNS stimulants for TBI-associated narcolepsy, post-traumatic hypersomnia and excessive daytime sleepiness requires further study with larger numbers of patients to determine effectiveness and benefit in this population.
Future research with multiple collaborating centres should attempt to delineate the pathophysiology of TBI-associated sleep disorders, including CNS-derived hypersomnia and circadian rhythm disturbances, and determine definitive, effective treatment for associated sleep disorders.
Similar content being viewed by others
1. Introduction
Traumatic brain injury (TBI) is a major cause of death among civilians and military personnel, as a consequence of motor vehicle accidents and explosive devices. Every year in the US, around 1.4 million civilians sustain TBI, of whom 124 000 develop long-term disability.[1] Presently, there are over 3.3 million Americans living with long-term disability related to TBI.[2] According to a recent systematic review, an estimate of the aggregate annual incidence of fatal TBI in the EU was approximately 235 per 100 000,[3] although substantial variation was seen among the member nations of the EU. A prospective study of hospitalized patients in the New South Wales state of Australia found that approximately 4 per 100 000 deaths were caused by CNS-related injuries associated with high-energy trauma, while 6.4 deaths per 100 000 were caused by CNS injuries from low-energy trauma.[4] A report from the Japanese trauma network indicated that the incidence of TBI was 27 per 100 000,[5] while more recent studies from Turkey and Malaysia report a much higher incidence of TBI in their populations.[6,7]
Recent studies have revealed a high prevalence of sleep disorders in the TBI population including narcolepsy, post-traumatic hypersomnia (PTH), periodic limb movements in sleep (PLMS) and obstructive sleep apnoea (OSA).[8–15] These disorders can have serious consequences on the overall well-being of the patient. Subjective complaints of ‘sleeping problems’ are common, and have been reported in 47% of 639 patients presenting to a minor head injury clinic.[16] Sleep, and especially rapid eye movement (REM) sleep, is believed to be involved with learning and consolidation of memories,[17–19] even with the development of insight.[20] Sleep loss/disruption may have a deleterious effect on hippocampal neurons involved in memory formation.[21,22] Cognitive deficits may result from sleep disturbances[23] and these may worsen the impairment in TBI.[8]
Sleep disorders that can affect daytime function such as insomnia, narcolepsy, shift-work sleep disorder and OSA are significant risk factors for motor vehicle accidents and can increase the risk of TBI.[24–27] Thus, there appears to be a complex relationship between disorders of sleep and TBI. Part of the confusion stems from the high risk of accidents among those with sleep disorders, especially sleep apnoea.[28–30] There is often a question about the existence of a sleep disorder prior to TBI, which may have been the underlying cause of the accident in the first place. Despite this complex relationship, we cannot discount the increased prevalence of sleep disorders, including sleep-disordered breathing (SDB), in patients with TBI who were asymptomatic prior to injury.
This review presents the epidemiology, pathophysiology and management of various sleep disorders that have been described in association with TBI.
2. Epidemiology of Sleep Disorders in Traumatic Brain Injury
2.1 Hypersomnia
Subjective excessive daytime sleepiness (EDS) can be estimated by the Epworth Sleepiness Scale (ESS). The ESS is a self-administered eight-item questionnaire to assess subjective daytime sleepiness in which the subject rates from 0 to 3 the likelihood of dozing in specific situations.[31,32] The objective measure of daytime sleepiness used is the Multiple Sleep Latency Test (MSLT).[33] This a series of four to five naps at 2-hour intervals of 20 minutes duration after a normal night of sleep documented by nocturnal polysomnography. Subjects sleep with EEG leads in place and sleep stages are scored in 30-second epochs. The mean sleep latency over these naps is the MSLT score. EDS is defined as an MSLT score of <10 minutes and pathological sleepiness as an MSLT score of <5 minutes.
EDS, defined as a score of >10 on the ESS, was found in 10.9% of a cohort of young and middle-aged healthy subjects.[34] Other estimates of questionnaire-based daytime sleepiness range from 5% to 15% in the general population.[35] Multiple studies have resulted in a general conclusion that EDS is common after TBI.[9,10,36] In a prospective multicentre study of 87 TBI patients,[9] EDS was found in 25%, while in an earlier case series of 71 patients with TBI,[36] 47% of subjects had EDS and 18.3% of those patients had pathological sleepiness as measured by the MSLT score. In a more recent retrospective study,[10] where overnight polysomnographic data were obtained in 54 patients with TBI, EDS was reported by 52% of all patients as documented by an ESS score of >11. Fifty-three percent (n = 15) of the 28 subjects with an ESS score of >11 who underwent MSLT had a sleep-onset latency of <5 minutes, suggestive of severe hypersomnia.
2.1.1 Hypersomnia Related to CNS Pathology
Narcolepsy
Case reports of ‘post-traumatic narcolepsy’ were published as early as 1941,[37] but it was impossible at the time to distinguish other sleep disorders such as sleep apnoea and narcolepsy from what is today known as PTH.[38] It is possible that narcolepsy could have preceded the head trauma or even contributed to it. On the other hand, a brain injury that is not of a traumatic nature such as irradiation of pituitary tumour has also been reported to cause narcolepsy with cataplexy.[39] Thus, it is conceivable that TBI can trigger events to unmask the disease in subjects who are at risk for developing it. In a retrospective study by Verma et al.,[10] the authors reported that 32% (9 of 28) of their subjects with TBI had severe hypersomnia (MSLT score of ≤5 minutes) and two or more episodes of sleep-onset REM periods (SOREMPs) compatible with a clinical diagnosis of narcolepsy. Only two patients in this group were positive for HLA-DRB1-15 and DQB1*0602 antigens, while this is found in 85% of those with idiopathic narcolepsy and cataplexy.[40] In a prospective multicentre study,[9] we found that 6% of TBI patients with a mean sleep latency of <10 minutes on the MSLT met the diagnostic criteria for narcolepsy, which is significantly higher than the sporadic form of the disease in the general population (0.056%).[41]
Post-Traumatic Hypersomnia
PTH is a disorder of excessive sleepiness that occurs as a result of a traumatic event involving the CNS.[42] The diagnosis of PTH is made when the following criteria are met: (i) sleepiness begins only after TBI; (ii) other causes of sleepiness are excluded by history and nocturnal polysomnography; and (iii) the MSLT score is <10 minutes with less than two SOREMPs.[43] It is important to exclude all other causes of hypersomnia in patients with TBI, including the sedative effects of medications such as antiepileptic drugs frequently used in this population.[15] In a retrospective study of 184 patients presenting to an outpatient sleep clinic with EDS after head and neck trauma, 49% had PTH.[44] A study of brain-injured patients in an inpatient rehabilitation facility found that 30% of these subjects had PTH.[36] In the only prospective multicentre study that has evaluated unselected TBI patients with nocturnal polysomnography and the MSLT, the prevalence of PTH was 11%.[9]
2.1.2 Hypersomnia Related to Sleep-Disordered Breathing
The syndrome of SDB encompasses a group of breathing disorders in sleep of varying severity such as OSA, upper airway resistance syndrome and central sleep apnoea.[45,46]
The most clinically important and the most widely studied SDB in patients with TBI is OSA. This is caused by narrowing or complete occlusion of the upper airway accompanied by continued attempts to breathe against this increased resistance. This results in intermittent hypoxemia, sleep disruption and EDS or difficulty sleeping.
Multiple studies with varying study designs have estimated that 23–70% of patients with TBI have SDB,[9,10,44,47,48] which is significantly higher than the expected prevalence in the population.[49] However, the true prevalence is likely closer to 23% as estimated by a prospective multicentre cohort study.[9] Cognitive deficits most commonly involving vigilance, attention, arousal, memory and executive functions have been associated with OSA.[23] It is also well established that cognitive impairment is associated with TBI and increases with increasing severity of injury.[50] Patients with TBI and OSA have a greater impairment of neurocognitive functions, especially of memory and sustained attention, as compared to TBI patients without SDB.[8]
2.2 Insomnia
Insomnia is defined as a repeated difficulty with sleep initiation, duration, consolidation or quality that occurs despite adequate time and opportunity for sleep, and results in some form of daytime impairment.[51] A complaint of sleep disruption over the previous year was reported in 30% of the general population, while 10% of these individuals had accompanying symptoms of daytime impairment.[52,53]
Even though EDS is a very important chronic symptom after TBI, hospitalized patients with a recent episode of TBI are more likely to complain of difficulty in initiating or maintaining sleep that can persist after hospital discharge.[54] Even minor head injury can be associated with decreased sleep quality and an increase in sleep interruptions as compared to the pre-injury state.[55,56] It appears that at least 30–50% of patients with TBI in the setting of outpatient rehabilitation centres have difficulty sleeping, of whom 64% complain of early morning awakenings and around 45% have problems initiating sleep.[11,57,58] When compared to subjects who have had orthopaedic injuries[59] or other non-neurological injuries,[60] patients with TBI are more likely to have difficulty initiating and maintaining sleep.
It appears that insomnia after TBI is associated with milder forms of brain injury in the presence of pain or depression.[60,61] Assault, as a causal mechanism of head injury, can in itself influence the effect of TBI on sleep. In a questionnaire study of patients with mild head injury where personal assault was the second most common cause of TBI (44%), 46% of patients complained of sleep disturbance that persisted at 6 months.[16] Despite the disproportionately high dropout rate among patients with TBI secondary to assault, this underscores the possibility that the additional psychological trauma of being personally attacked may contribute to insomnia as distinct from a head injury sustained in a road traffic accident.[11]
Even though, patients with TBI report poor-quality sleep, polysomnographic findings from a study[12] of ten patients with TBI and age- and sex-matched controls showed comparable sleep-onset latency, arousal index and sleep efficiency between the two groups. However, patients with TBI had significantly more awakenings, concordant with their self-reported symptoms noted on an earlier study.[11] These findings are consistent with those of an earlier study of 14 patients with mild to severe TBI compared to healthy controls,[62] which documented a disparity between subjective and objective measures of insomnia in these patients. The majority of the TBI patients (71.4%) in this sample had subjective insomnia by questionnaires. Of the five subjects reporting good sleep quality, three had objective (polysomnographic) evidence of insomnia as defined by sleep-onset latency or a wake after sleep onset of over 30 minutes. Of the four patients with objective evidence of good-quality sleep, two met criteria for insomnia by questionnaires. Similar to hypersomnia, subjective complaints of insomnia may not be reliable to affirm the diagnosis of insomnia in this population.
2.3 Circadian Rhythm Disorders
The literature is lacking in prospective studies specifically designed to describe the epidemiology of circadian rhythm disturbances after TBI. However, there have been published reports that suggest the existence of post-traumatic delayed-sleep phase syndrome (DSPS).[63–65] A case report of a patient with a non-24-hour sleep-wake cycle (hypernyctohemeral) syndrome as a late complication of TBI has been reported.[66] Usually, a minority of patients presenting with insomnia in the general population have a circadian rhythm disorder,[67] but in a recent study by Ayalon and colleagues,[68] 36% of patients with minor TBI, who were referred for evaluation of insomnia, were diagnosed with a circadian rhythm disorder. Of these individuals, 52% had DSPS and the rest had an irregular sleep-wake pattern (ISWP). Three patients in the ISWP group had a decrease in the amplitude of the oral temperature cycle as compared to the DSPS group. In another study by Steele and colleagues,[69] ten patients with DSPS post-acute TBI had similar habitual sleep times (assessed by Morningness-Eveningness Questionnaire) and onset of melatonin secretion as compared to their controls. Conflicting results from the two studies could have resulted from lack of statistical power from a small sample size and/or the inherent differences between the pathophysiological processes associated with nature and timing of the injury. Future studies are necessary to better describe the nature of circadian rhythm disorder associated with TBI.
2.4 Periodic Limb Movements in Sleep
PLMS is a movement disorder of sleep characterized by slow and rhythmic movements of predominantly lower limbs (although upper limb movements may also occur) during non-REM sleep. Approximately 80% of patients with restless legs syndrome (RLS) also have PLMS,[70] but RLS is a disorder of wakefulness characterized by a subjective feeling of discomfort accompanied by a need to move the extremities. This occurs more during the evening and night and may cause sleep-onset insomnia. Periodic limb movements (PLMs), on the other hand, occur during sleep and may be a cause for sleep-maintenance insomnia. PLMs are rhythmic anterior tibialis contractions of 0.5–5 seconds duration in groups of at least four contractions interrupted by 5–90 seconds of quiescence.[71] Currently, more than 15 PLMs per hour (PLM index, PLMI) is considered abnormal in adults, while an index of >5 PLMs per hour is considered abnormal in children. All of the current studies on TBI have used the older criteria of PLMI ≥5 per hour as the minimal criteria for the diagnosis of PLMS. The proportion of institutionalized TBI patients with PLMS was reported to be as high as 25.4%,[36] while in a more recent multicentre study of both institutionalized and ambulatory TBI patients the prevalence of PLMS was 7%.[9]
2.5 Other Movement Disorders and Parasomnias
There is scant literature describing the prevalence of parasomnias associated with TBI, but the one most commonly reported is REM sleep behaviour disorder (RBD). Clinical RBD is characterized by dream enactment and the presence of REM sleep without normally occurring atonia. Subclinical RBD is the descriptive term for the polysomnographic detection of excessive electromyogram tone during REM sleep and/or movement activity without overt dream enactment.[72] In a recent study, parasomnias were the presenting complaint in 25% of patients with TBI, of which clinical or subclinical RBD was the most common, having been found in 7 of 54 (13%) patients with sleep complaints.[10]
Sleep-related bruxism is also known as nocturnal tooth grinding.[72] Since bruxism is fairly common in the general population, it is not surprising that this should also be found in patients with TBI, but there are no reliable studies that have measured the prevalence of bruxism in this population.
Jactatio nocturna or head banging is a sleep-related rhythmic movement disorder rarely seen in adults. There is one report of the onset of jactatio nocturna after TBI in an adult patient who also had global encephalopathy related to the injury.[73]
2.6 Dreams
There have been conflicting reports with regard to potentially reduced REM sleep in patients with minor TBI.[74,75] Even though early studies including case series have reported a decrease in dreaming among patients post-TBI,[76,77] a more recent study based on patient questionnaires[78] reported a change in the quality rather than the quantity of dreams. In 51 subjects with TBI, frequent dreaming with threatening content increased by 23.5%, while dreams with sexual content decreased by 9.8% after TBI.
3. Pathophysiology
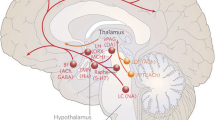
While there have been no studies to date that have described a localizing neuroanatomical injury as a predictor of hypersomnia in patients with TBI, there is reason to believe that hypothalamic injury with decreased levels of wake-promoting neurotransmitters such as hypocretin (orexin-A) and histamine may be involved in the pathophysiology of EDS associated with TBI. Early studies have shown that hypothalamic damage appears to be common in TBI patients.[79,80] Low CSF levels of both hypocretin and histamine are associated with hypersomnia.[81] Measured levels of CSF hypocretin were low (<320 pg/mL) in 95% of 44 patients with acute TBI.[82] The lowest levels of CSF hypocretin were found in patients who were comatose. When studied 6 months post-injury, 4 of the 14 patients with EDS had low hypocretin levels (289 ± 64 pg/mL), while those without EDS had normal levels (444 ± 113 pg/mL; p = 0.05).[14]
The role of CSF histamine as a diagnostic predictor in PTH is less clear. A recent study reported low CSF histamine levels in idiopathic hypersomnia but not in patients with OSA.[81] Low CSF histamine levels have also been reported in patients with narcolepsy with or without cataplexy and with or without low CSF hypocretin levels.[83] Since histamine in the CNS is produced largely by the tuberomammillary nucleus,[84,85] this suggests an area to be further explored in future investigations in patients with TBI. The relationship between narcolepsy and TBI is still controversial, since this is generally regarded as a disease with a genetic basis and presumed to be present prior to injury. Case reports of post-traumatic narcolepsy have been published as early as 1941.[37] However, these reports do not have the necessary components of polysomnography to make a confident diagnosis of narcolepsy. On the other hand, more recent investigators have questioned whether narcolepsy after TBI is ‘unmasked’ in subjects who have pre-existing risk factors.[86]
The relationship between SDB and TBI is complex. Pre-existing untreated SDB associated with hypersomnia can predispose individuals to motor vehicle accidents, which can lead to TBI. However, injury to upper respiratory muscles along with TBI can cause post-traumatic OSA.[47] It is also important to consider factors such as weight gain, supine sleep and medication effects such as reduced muscle tone and respiratory depression that can predispose TBI patients to SDB.
Insomnia may develop in TBI patients as a result of ‘psychological trauma’ resulting from personal assaults and accidents resulting in TBI. Psychophysiological insomnia may arise in the context of the recovery period after TBI and persist long after discharge from the acute care hospital.
The development of circadian rhythm disorders after TBI can be explained by one of two mechanisms. The first would be injury to the suprachiasmatic nucleus or other thalamic structures. While this is possible, there is no evidence to support that mechanism in reports to date. The second possibility is that sleep/wake disturbances arise as a result of prolonged hospitalization, loss of Zeitgebers and use of sedative-hypnotics and analgesics following TBI. This can be seen in other hospitalized patients, especially in intensive care units and more so after mechanical ventilation. However, prolonged hospitalization as a possible mechanism for circadian rhythm disorders in all patients with TBI is debatable. A study of 42 patients with mild TBI evaluated for insomnia in an outpatient clinic revealed that, while 36% of those subjects had a circadian rhythm disorder,[68] prolonged hospitalization did not play a significant role in the pathophysiology of these cases.
4. Management
4.1 Diagnosis
Based upon the high prevalence of sleep disorders in TBI such as SDB and PTH,[9] and the fact that SDB associated with TBI contributes to additional impairment,[8] we recommend that patients with TBI should undergo nocturnal polysomnography followed by the MSLT. The MSLT may be cancelled if significant SDB is found on nocturnal polysomnography, but is necessary for the diagnosis of narcolepsy and PTH. Since the correlation between objective measurement of sleepiness by the MSLT and subjective sleepiness score by the ESS is poor in TBI subjects, patient selection for nocturnal polysomnography based on the ESS can be unreliable.[9,36]
The diagnosis of insomnia is usually weighted by the historical information obtained from patients, family members or caregivers.[52] However, in patients with TBI, due to the poor predictive value of subjective measures and a high prevalence of SDB, PLMS and circadian rhythm disorders[9] that can also present with insomnia, further testing using nocturnal polysomnography is necessary to detect these co-morbid sleep disorders. It should be noted, however, that polysomnographic characterization of sleep architecture has not been systematically evaluated in this population.
Actigraphy and/or sleep logs are crucial in the diagnosis and management of circadian rhythm disorders associated with TBI.
4.2 Treatment
SDB in this population is effectively treated with continuous positive airway pressure therapy, which is titrated in the sleep laboratory with polysomnography until all apnoea, hypopneas and respiratory effort-related arousals are eliminated.[87] It should be noted that despite elimination of SDB with continuous positive airway pressure, reversal of hypersomnia and neurocognitive deficits may not follow in this population.[87] This could be due to persisting PTH after treatment of co-morbid SDB.
The data on the efficacy of modafinil in the treatment of PTH and narcolepsy after TBI are equivocal at a dose of 200 mg/day.[87] This is due to the variable response seen in a small sample size. It is possible that higher doses of modafinil or other medications such as armodafinil (150–250 mg/day), methylphenidate (5–60 mg/day) or dextroamphetamine (5–60 mg/day) may be of benefit. Modafinil in doses up to 400 mg/day failed to show improvement in fatigue and subjective sleepiness in a larger group of patients with TBI, but these patients did not undergo polysomnography and exclusion of SDB.[88]
Cognitive behavioural therapy appears to be of benefit in TBI patients with insomnia[89] and has also been shown to improve the emotional well-being of these patients.[90] Treatment of post-TBI insomnia with hypnotics has not been systematically studied, even though as many as 20% of TBI patients may receive such treatment.[91] Currently used medications include the melatonin receptor agonist ramelteon and the nonbenzodiazepine hypnotics that act on the Ω-1 benzodiazepine receptor subtype located in the GABAA receptor complex: zolpidem, zaleplon, zopiclone and eszopiclone. These are all indicated for sleep-onset insomnia, but zaleplon has an onset of action of 14–30 minutes and a short, 1-hour half-life that enables its use in the middle of the night as needed.[92] Eszoplicone has a long half-life of 6 hours and is approved for long-term use, as well as sleep-maintenance insomnia. Ramelteon is also approved for long-term use in chronic insomnia, and has no potential to worsen SDB. Zolpidem has an onset of action of 30 minutes and a half-life of 1.5–4.5 hours, with an extended-release formulation available. It has been associated with cognitive impairment,[93] and also with parasomnias such as sleep walking.[94] Zopiclone has a half-life of 3–6 hours and is not available in the US. Benzodiazepine hypnotics are not recommended for routine use after TBI.[95]
Even though the administration of methylphenidate did not have adverse effects, it was not shown to have significant benefit in an attempt to improve the sleep/wake cycle of patients in an inpatient brain injury unit.[96] Bright-light therapy (10 000 lux) and melatonin (0.1–0.5 mg/night), useful in the treatment of circadian rhythm disorders, have not been systematically studied in TBI patients. At this time, there is no literature to support the use of ramelteon in circadian rhythm disorders.
Bruxism can be treated with a tooth guard when feasible to prevent permanent damage to dentition. Successful treatment of bruxism with botulinum toxin-A in a patient with TBI has been reported.[97]
Pramipexole at a dose of 0.375 mg/day was effective in the treatment of PLMS after TBI in one prospective study, but only 4 of the 57 TBI patients studied had PLMS.[87] In non-TBI patients, PLMS is treated with pramipexole at 0.125–0.5 mg/day or ropinirole at 0.25–4 mg/day.
Imipramine was used successfully to resolve jactatio nocturna in one patient with TBI.[73]
5. Conclusion
It is clear that all TBI patients are at considerable risk for various sleep disorders, the most common being OSA, PTH, narcolepsy, circadian rhythm disorders and insomnia. Because of the unreliability of subjective sleep symptoms in these patients, objective testing with nocturnal polysomnography and the MSLT are necessary to diagnose co-morbid sleep disorders. Future studies must systematically evaluate new and existing treatment modalities for these disorders in TBI patients with meaningful outcome measures and long-term follow-up.
References
Selassie AW, Zaloshnja E, Langlois JA, et al. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil 2008 Mar–Apr; 23(2): 123–31
Zaloshnja E, Miller T, Langlois JA, et al. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil 2008 Nov–Dec; 23(6): 394–400
Tagliaferri F, Compagnone C, Korsic M, et al. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 2006 Mar; 148(3): 255–68; discussion 268
Evans JA, van Wessem KJ, McDougall D, et al. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg 2010 Jan; 34(1): 158–63
Sheishi T, Shozaburo U, Tatsushi Y, et al. Kumamoto traumatic coma databank: past, present and future. Neurotraumatology 1998; 21(2): 118–24
Sabariah FJ, Ramesh N, Mahathar AW. National Trauma Database (NTrD): improving trauma care: first year report. Med J Malaysia 2008 Sep; 63 Suppl. C: 45–9
Tokdemir M, Kafadar H, Turkoglu A, et al. Comparison of the severity of traumatic brain injuries in pedestrians and occupants of motor vehicles admitted to Firat health center: a five-year series in an Eastern Turkish city. Med Sci Monit 2009 Jan; 15(1): PI1–4
Wilde MC, Castriotta RJ, Lai JM, et al. Cognitive impairment in patients with traumatic brain injury and obstructive sleep apnea. Arch Phys Med Rehabil 2007 Oct; 88(10): 1284–8
Castriotta RJ, Wilde MC, Lai JM, et al. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med 2007 Jun 15; 3(4): 349–56
Verma A, Anand V, Verma NP. Sleep disorders in chronic traumatic brain injury. J Clin Sleep Med 2007 Jun 15; 3(4): 357–62
Parcell DL, Ponsford JL, Rajaratnam SM, et al. Self-reported changes to nighttime sleep after traumatic brain injury. Arch Phys Med Rehabil 2006 Feb; 87(2): 278–85
Parcell DL, Ponsford JL, Redman JR, et al. Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: a preliminary study. Arch Phys Med Rehabil 2008 May; 89(5): 843–50
Rao V, Spiro J, Vaishnavi S, et al. Prevalence and types of sleep disturbances acutely after traumatic brain injury. Brain Inj 2008 May; 22(5): 381–6
Baumann CR, Werth E, Stocker R, et al. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain 2007 Jul; 130 (Pt 7): 1873–83
Watson NF, Dikmen S, Machamer J, et al. Hypersomnia following traumatic brain injury. J Clin Sleep Med 2007 Jun 15; 3(4): 363–8
Haboubi NH, Long J, Koshy M, et al. Short-term sequelae of minor head injury (6 years experience of minor head injury clinic). Disabil Rehabil 2001 Sep 20; 23(14): 635–8
Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. Trends Neurosci 2001 Apr; 24(4): 237–43
Rauchs G, Bertran F, Guillery-Girard B, et al. Consolidation of strictly episodic memories mainly requires rapid eye movement sleep. Sleep 2004 May 1; 27(3): 395–401
Stickgold R. Sleep-dependent memory consolidation. Nature 2005 Oct 27; 437(7063): 1272–8
Wagner U, Gais S, Haider H, et al. Sleep inspires insight. Nature 2004 Jan 22; 427(6972): 352–5
McDermott CM, LaHoste GJ, Chen C, et al. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci 2003 Oct 22; 23(29): 9687–95
Ruskin DN, Liu C, Dunn KE, et al. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci 2004 Jun; 19(11): 3121–4
Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc 2004 Sep; 10(5): 772–85
Daley M, Morin CM, Leblanc M, et al. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med 2009 Apr; 10(4): 427–38
Rodenstein D. Driving in Europe: the need of a common policy for drivers with obstructive sleep apnoea syndrome. J Sleep Res 2008 Sep; 17(3): 281–4
Stone KL, Ancoli-Israel S, Blackwell T, et al. Actigraphy-measured sleep characteristics and risk of falls in older women. Arch Intern Med 2008 Sep 8; 168(16): 1768–75
Stradling J. Driving and obstructive sleep apnoea. Thorax 2008 Jun; 63(6): 481–3
Ellen RL, Marshall SC, Palayew M, et al. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med 2006 Apr 15; 2(2): 193–200
Leger D. The cost of sleep-related accidents: a report for the National Commission on Sleep Disorders Research. Sleep 1994 Feb; 17(1): 84–93
Young T, Blustein J, Finn L, et al. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997 Aug; 20(8): 608–13
Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991 Dec; 14(6): 540–5
Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest 1993 Jan; 103(1): 30–6
Carskadon MA, Dement WC, Mitler MM, et al. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep 1986 Dec; 9(4): 519–24
Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep 1997 Oct; 20(10): 844–9
Partinen M, Hublin C. Epidemiology of sleep disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia (PA): Saunders, 2005: 626–47
Masel BE, Scheibel RS, Kimbark T, et al. Excessive daytime sleepiness in adults with brain injuries. Arch Phys Med Rehabil 2001 Nov; 82(11): 1526–32
Gill AW. Idiopathic and traumatic narcolepsy. Lancet Neurol 1941; 1: 474–6
American Academy of Sleep Medicine. International classification of sleep disorders. 2nd ed. Diagnostic and coding manual. Westchester (IL): American Academy of Sleep Medicine, 2005: 79–116
Dempsey OJ, McGeoch P, de Silva RN, et al. Acquired narcolepsy in an acromegalic patient who underwent pituitary irradiation. Neurology 2003 Aug 26; 61(4): 537–40
Mignot E, Hayduk R, Black J, et al. HLA DqB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep 1997; 20: 1012–20
Silber MH, Krahn LE, Olson EJ, et al. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002 Mar 15; 25(2): 197–202
American Academy of Sleep Medicine. International classification of sleep disorders, revised. Diagnostic and coding manual. Chicago (IL): American Academy of Sleep Medicine, 2001
Castriotta RJ. Brain and spinal cord injury. In: Lee-Chiong T, editor. Sleep: a comprehensive handbook. Hoboken (NJ): John Wiley & Sons, Inc., 2006: 817–21
Guilleminault C, Yuen KM, Gulevich MG, et al. Hypersomnia after head-neck trauma: a medicolegal dilemma. Neurology 2000 Feb 8; 54(3): 653–9
American Academy of Sleep Medicine. International classification of sleep disorders. 2nd ed. Diagnostic and coding manual. Westchester (IL): American Academy of Sleep Medicine, 2005: 33–77
Hoffstein V. Snoring and upper airway resistance. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia (PA): Elsevier Saunders, 2005: 1001–12
Castriotta RJ, Lai JM. Sleep disorders associated with traumatic brain injury. Arch Phys Med Rehabil 2001 Oct; 82(10): 1403–6
Webster JB, Bell KR, Hussey JD, et al. Sleep apnea in adults with traumatic brain injury: a preliminary investigation. Arch Phys Med Rehabil 2001 Mar; 82(3): 316–21
Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002 May 1; 165(9): 1217–39
Dikmen S, Machamer J, Temkin N, et al. Neuropsychological recovery in patients with moderate to severe head injury: 2 year follow-up. J Clin Exp Neuropsychol 1990 Aug; 12(4): 507–19
American Academy of Sleep Medicine. International classification of sleep disorders. 2nd ed. Diagnostic and coding manual. Westchester (IL): American Academy of Sleep Medicine, 2005
[No authors listed]. NIH state of the science conference statement on manifestations and management of chronic insomnia in adults. J Clin Sleep Med 2005 Oct 15; 1 (4): 412-21
Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep 1999 May 1; 22 Suppl. 2: S347–53
Cohen M, Oksenberg A, Snir D, et al. Temporally related changes of sleep complaints in traumatic brain injured patients. J Neurol Neurosurg Psychiatry 1992 Apr; 55(4): 313–5
Kaufman Y, Tzischinsky O, Epstein R, et al. Long-term sleep disturbances in adolescents after minor head injury. Pediatr Neurol 2001 Feb; 24(2): 129–34
Parsons LC, Ver Beek D. Sleep-awake patterns following cerebral concussion. Nurs Res 1982 Sep-Oct; 31(5): 260–4
Fichtenberg NL, Zafonte RD, Putnam S, et al. Insomnia in a post-acute brain injury sample. Brain Inj 2002 Mar; 16(3): 197–206
Clinchot DM, Bogner J, Mysiw WJ, et al. Defining sleep disturbance after brain injury. Am J Phys Med Rehabil 1998 Jul–Aug; 77(4): 291–5
Perlis ML, Artiola L, Giles DE. Sleep complaints in chronic postconcussion syndrome. Percept Mot Skills 1997 Apr; 84(2): 595–9
Beetar JT, Guilmette TJ, Sparadeo FR. Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch Phys Med Rehabil 1996 Dec; 77(12): 1298–302
Fichtenberg NL, Millis SR, Mann NR, et al. Factors associated with insomnia among post-acute traumatic brain injury survivors. Brain Inj 2000 Jul; 14(7): 659–67
Ouellet MC, Morin CM. Subjective and objective measures of insomnia in the context of traumatic brain injury: a preliminary study. Sleep Med 2006 Sep; 7(6): 486–97
Patten SB, Lauderdale WM. Delayed sleep phase disorder after traumatic brain injury. J Am Acad Child Adolesc Psychiatry 1992 Jan; 31(1): 100–2
Nagtegaal JE, Kerkhof GA, Smits MG, et al. Traumatic brain injury-associated delayed sleep phase syndrome. Funct Neurol 1997 Nov–Dec; 12(6): 345–8
Quinto C, Gellido C, Chokroverty S, et al. Posttraumatic delayed sleep phase syndrome. Neurology 2000 Jan 11; 54(1): 250–2
Boivin DB, Caliyurt O, James FO, et al. Association between delayed sleep phase and hypernyctohemeral syndromes: a case study. Sleep 2004 May 1; 27(3): 417–21
Weitzman ED, Czeisler CA, Coleman RM, et al. Delayed sleep phase syndrome: a chronobiological disorder with sleep-onset insomnia. Arch Gen Psychiatry 1981 Jul; 38(7): 737–46
Ayalon L, Borodkin K, Dishon L, et al. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology 2007 Apr 3; 68(14): 1136–40
Steele DL, Rajaratnam SM, Redman JR, et al. The effect of traumatic brain injury on the timing of sleep. Chronobiol Int 2005; 22(1): 89–105
Montplaisir J, Boucher S, Poirier G, et al. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord 1997 Jan; 12(1): 61–5
American Academy of Sleep Medicine. International classification of sleep disorders. 2nd ed. Diagnostic and coding manual. Westchester (IL): American Academy of Sleep Medicine, 2005: 182–6
American Academy of Sleep Medicine. International classification of sleep disorders. 2nd ed. Diagnostic and coding manual. Westchester (IL): American Academy of Sleep Medicine, 2005: 148–51
Drake MEJ. Jactatio nocturna after head injury. Neurology 1986 Jun; 36(6): 867–8
Gosselin N, Lassonde M, Petit D, et al. Sleep following sport-related concussions. Sleep Med 2009 Jan; 10(1): 35–46
Schreiber S, Barkai G, Gur-Hartman T, et al. Long-lasting sleep patterns of adult patients with minor traumatic brain injury (mTBI) and non-mTBI subjects. Sleep Med 2008 Jul; 9(5): 481–7
Humphrey ME, Zangwill OL. Cessation of dreaming after brain injury. J Neurol Neurosurg Psychiatry 1951; 14(4): 322–5
Prigatano GP, Stahl ML, Orr WC, et al. Sleep and dreaming disturbances in closed head injury patients. J Neurol Neurosurg Psychiatry 1982 Jan; 45(1): 78–80
Benyakar M, Tadir M, Groswasser Z, et al. Dreams in head-injured patients. Brain Inj 1988 Oct-Dec; 2(4): 351–6
Crompton MR. Brainstem lesions due to closed head injury. Lancet 1971 Apr 3; 1(7701): 669–73
Crompton MR. Hypothalamic lesions following closed head injury. Brain 1971; 94(1): 165–72
Kanbayashi T, Kodama T, Kondo H, et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep 2009 Feb 1; 32(2): 181–7
Baumann CR, Stocker R, Imhof HG, et al. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology 2005 Jul 12; 65(1): 147–9
Nishino S, Sakurai E, Nevsimalova S, et al. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep 2009 Feb 1; 32(2): 175–80
Watanabe T, Taguchi Y, Shiosaka S, et al. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res 1984 Mar 12; 295(1): 13–25
Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A 1984 Apr; 81(8): 2572–6
Lankford DA, Wellman JJ, O’Hara C. Posttraumatic narcolepsy in mild to moderate closed head injury. Sleep 1994 Dec; 17(8 Suppl): S25–8
Castriotta RJ, Atanasov S, Wilde MC, et al. Treatment of sleep disorders after traumatic brain injury. J Clin Sleep Med 2009; 5(2): 137–44
Jha A, Weintraub A, Allshouse A, et al. A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J Head Trauma Rehabil 2008 Jan-Feb; 23(1): 52–63
Ouellet MC, Morin CM. Cognitive behavioral therapy for insomnia associated with traumatic brain injury: a single-case study. Arch Phys Med Rehabil 2004 Aug; 85(8): 1298–302
Bradbury CL, Christensen BK, Lau MA, et al. The efficacy of cognitive behavior therapy in the treatment of emotional distress after acquired brain injury. Arch Phys Med Rehabil 2008 Dec; 89(12 Suppl. ): S61–8
Worthington AD, Melia Y. Rehabilitation is compromised by arousal and sleep disorders: results of a survey of rehabilitation centres. Brain Inj 2006; 20(3): 327–32
Doghramji K. The need for flexibility in dosing of hypnotic agents. Sleep 2000; 23: S16–20
Scharf MD, Roth T, Vogel G. A multicenter placebo controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry 1994; 55: 192–9
Yanf W, Dollear M, Muthukrishnan SR. One rare side effect of zolpidem: sleepwalking. A case report. Arch Phys Med Rehabil 2005; 86: 1265–6
Flanagan SR, Greenwald B, Wieber S. Pharmacological treatment of insomnia for individuals with brain injury. J Head Trauma Rehabil 2007; 22(1): 67–70
Al-Adawi S, Burke DT, Dorvlo AS. The effect of methylphenidate on the sleep-wake cycle of brain-injured patients undergoing rehabilitation. Sleep Med 2006 Apr; 7(3): 287–91
Ivanhoe CB, Lai JM, Francisco GE. Bruxism after brain injury: successful treatment with botulinum toxin-A. Arch Phys Med Rehabil 1997 Nov; 78(11): 1272–3
Acknowledgements
Prof. Castriotta has received research support from Cephalon, Inc. for investigator-initiated research. Dr Murthy has no conflicts of interest to report. No financial support was received to prepare this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castriotta, R.J., Murthy, J.N. Sleep Disorders in Patients with Traumatic Brain Injury. CNS Drugs 25, 175–185 (2011). https://doi.org/10.2165/11584870-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11584870-000000000-00000